Abstract
Introduction Daratumumab is an anti-CD38 IgG1k monoclonal antibody with direct tumor killing and immunomodulatory mechanisms of action which was shown to have clinical benefits when added to the standard-of-care induction regimen in transplant eligible multiple myeloma (MM) patients. However, prior studies have indicated a potential reduction in stem cell yield in patients exposed to daratumumab prior to stem cell mobilization. Additionally, prior studies have not utilized combination mobilization regimens with G-CSF and the CXCR4 antagonist plerixafor as standard-of-care, which may overcome the deleterious effect of daratumumab. In this study, we investigated the stem cell yield of plerixafor-treated patients with daratumumab exposure compared to those without.
Methods We conducted a retrospective study of patients with MM at our institution who underwent autologous stem cell mobilization and collection during first-line treatment over a 15-month period from 2021-2022. Following induction, patients underwent mobilization with a regimen of G-CSF for 5 days with plerixafor on the 4th day. Circulating CD34+ stem cells were enumerated on the 5th day, after which patients underwent approximately 20L apheresis with a collection goal of 5x106 CD34+ cells/kg of body weight. The primary endpoint of this study was the comparison of circulating CD34+ cells between patients who received daratumumab during induction and those who did not. Secondary endpoints included collection CD34+ yield and proportion who met the collection goal.
Results 123 patients were included in the analysis. The median age was 65 (range: 34-77), 54% were male, and 84% were White. Twenty-two percent were stage I, 58% stage II, and 19% stage III by the Revised International Staging System.
Forty-seven percent received daratumumab during induction. Of those, the median number of cycles administered was 4 (range: 1-5). Ninety percent received daratumumab in combination with bortezomib, lenalidomide and dexamethasone. The two cohorts were balanced in terms of age, gender, race, and exposure to lenalidomide, alkylators and radiation during induction.
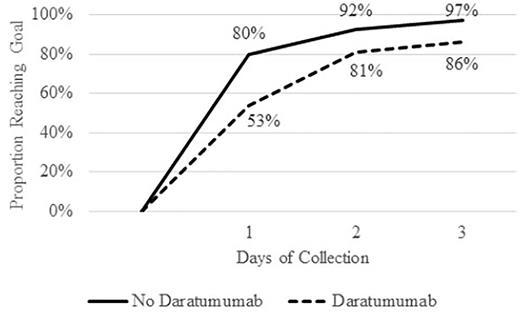
Patients with daratumumab exposure had a mean mobilization of 57.5 CD34+ cells/mcl compared to 96.4 for those without (p < 0.001). The mean CD34+ cell collection yield in 1 apheresis procedure was 6.0 x 106 cells/kg compared to 10.6 x 106 cells/kg (p < 0.001). Fifty-three percent of patients with daratumumab exposure met the collection goal compared to 80% of patients without (p < 0.001). Of the patients who did not meet the goal in one apheresis procedure, the majority were able to meet the goal with 1-3 additional days of apheresis (Figure 1). However, only 86% of patients with daratumumab exposure met the collection goal compared to 97% of those without (p < 0.001).
Ten patients failed to collect 2.0 x 106 cells/kg, the institutional minimum for transplantation, 9 of which received daratumumab during induction. Eight were able to collect the minimum with an additional 1-3 days of apheresis; 2 patients required an additional mobilization procedure and collection but were able to proceed to transplantation.
After adjusting for covariates including age, gender, race, weight, and exposure to lenalidomide, alkylators, and radiation, daratumumab exposure was associated with a 37.9 cells/mcl decrease in mean mobilization of CD34+ and 4.2 x 106 cells/kg decrease in CD34+ cell collection (both p < 0.001). Alkylator exposure was associated with a 43.3 cells/mcl decrease in mean mobilization of CD34+ (p = 0.040) and 4.1 x 106 cells/kg decrease in CD34+ cell collection (p = 0.057). Lenalidomide exposure was associated with a 103.4 cells/mcl decrease in mean mobilization of CD34+ (p <0.001) and 8.7 x 106 cells/kg decrease in CD34+ cell collection (p <0.001).
Conclusion Inclusion of daratumumab in the induction regimen for transplant-eligible patients with MM resulted in a significant reduction in stem cell mobilization and stem cell yield, even with the use of plerixafor-containing mobilization regimens. This reduction was similar to alkylator exposure but less than lenalidomide exposure. With the increasing use of quadruplet induction regimens, the frequency of mobilization failure is likely to increase in the future and new mobilization regimens and approaches are needed to guarantee adequate cell yields without the need for additional days of apheresis and/or mobilizations.
Disclosures
Vickroy:Janssen Advisory Board: Consultancy. Crees:BioLineRx Ltd.: Research Funding. Schroeder:Fortis: Research Funding; Cellect Inc: Research Funding; Genentech Inc: Research Funding; Incyte: Research Funding; Seagen Inc.: Research Funding; Amgen: Research Funding; Celgene: Research Funding. DiPersio:Amphivena Therapeutics: Research Funding; hC Bioscience, Inc.: Membership on an entity's Board of Directors or advisory committees; VLA-4 Inhibitor with Washington University and Magenta Therapeutics: Patents & Royalties; NeoImmune Tech: Research Funding; Incyte: Consultancy, Research Funding; WUGEN: Current equity holder in private company, Research Funding; RiverVest Venture Partners: Consultancy, Membership on an entity's Board of Directors or advisory committees; CAR-T cell Product with Washington University and WUGEN: Patents & Royalties; BioLineRx, Ltd.: Research Funding; Macrogenics: Research Funding; Magenta Therapeutics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees. Vij:GSK: Honoraria; Beigene: Honoraria; oncopetideslegend: Honoraria; Sanofi: Honoraria, Other: Grant support; BMS: Honoraria, Other: Grant support; Janssen: Honoraria; Takeda: Honoraria, Other: Grant support; adaptive: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal